Water Jelly Crystals – Crystal Growing Experiment
DIY Crystal Growing Kits for Kids
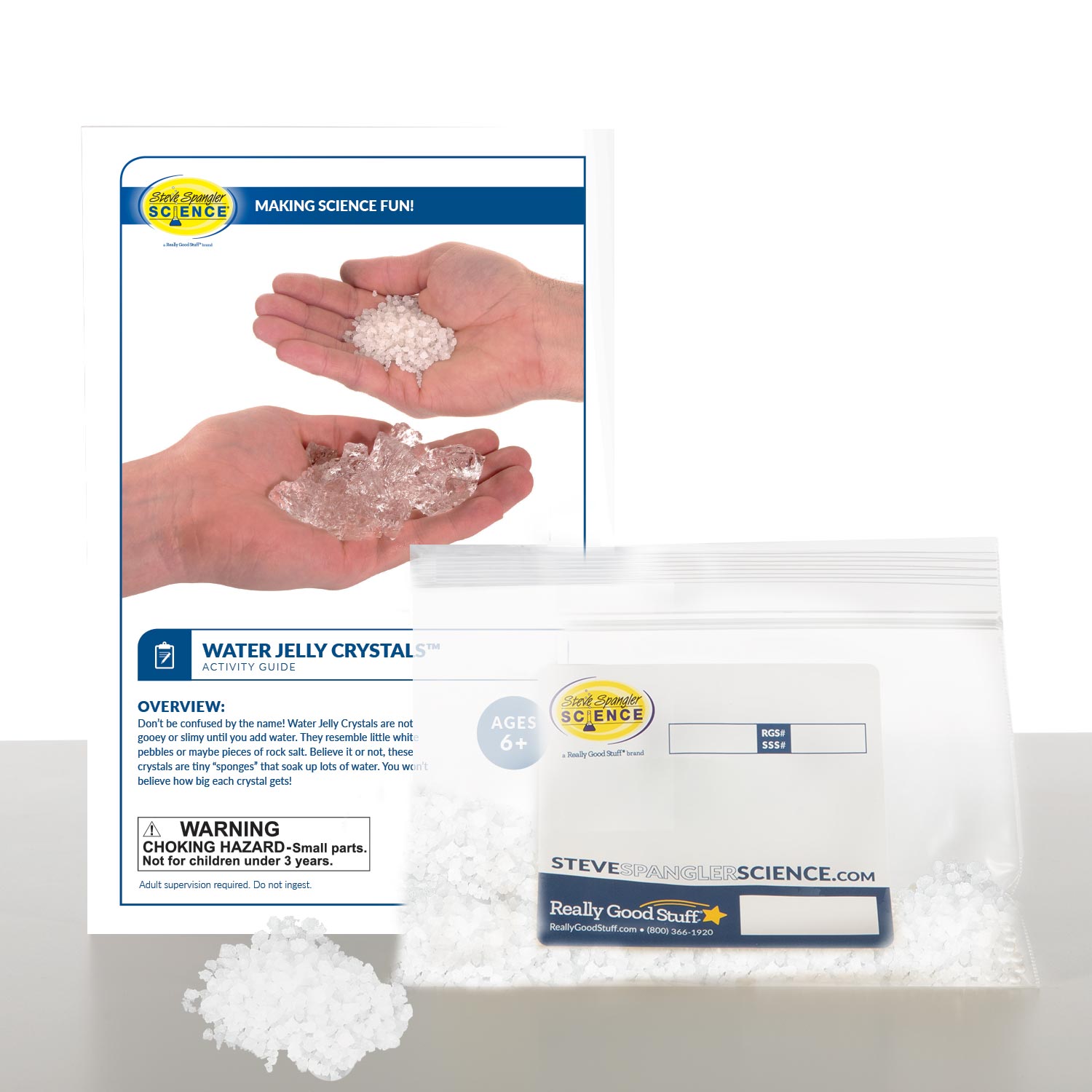
Water Jelly Crystals
In the Water Jelly Crystals experiment, you’ll start with little nuggets that look like rock salt and end up with huge, gleaming, water-filled blobs. Your discoveries using a superabsorbent polymer have just begun.
If you wore a modern diaper at one point in your infant life, then you’ve actually already had experience with one type of these chemical marvels. That’s right, superabsorbent polymers are used to lock moisture in baby diapers so that they don’t leak. The polymers we’ll be using in this experiment absorb water, too — a lot of water, in fact. The Water Jelly Crystals can be used not only for a lot of fun activities, but also for practical purposes.
Experiment Materials
- Water Jelly Crystals (available as clear or colors and in different quantities)
- Water (Distilled water is best but tap water works.)
- Paper towels
- Quart-sized, sealable plastic bags
- Measuring spoons
- A medium bowl
- Dinner plate
- Ruler
- Adult supervision
Experiment Videos
Experiment

1
NOTE: If you’re planning to have colored crystals, it’s easier to start with the color of water you want before you toss in the nuggets. Color Fizzer Tablets are best or food coloring can work. If this is your first time working with Water Jelly Crystals, don’t use colors so you can see what to expect. You can add color later!
Place 1 teaspoon of nuggets into a quart-sized plastic bag. A clear bag will make it easy to observe the experiment. Fill the bag with water and seal it tightly. Place the bag into a bowl to catch any slow leaks. Observe the crystals after 10 minutes, 30 minutes and 1 hour. What changes to the polymer do you see? Let the soaking continue overnight, then observe the crystals again to see what changes have occurred.

2
When you check the bag after a few hours, those tiny nuggets have grabbed onto water molecules and have exploded into huge, globby, Water Jelly Crystals as a result.
Wash your hands and then scoop a handful of the cool, shimmery lumps out of the bag. The crystals are fragile and can break, so make sure you handle them gently. The longer you soak the nuggets, the more water they’ll absorb.
3
Stir the crystals using your hand for an interesting sensation. You may think it’s cold and slimy, but leave your hand in the bag for a few minutes and do some testing. What changes do you notice?

4
Remove three or four Water Jelly Crystals from the bag and put them on a plate. Use a ruler to measure their size and compare one of the hydrated crystals to a dry nugget. How do the sizes of the water-filled crystals compare to each other? How do the dry nuggets compare to each other in size? What other differences, aside from size, do you notice?
5
Try to break a nugget into smaller pieces with dry fingers. Now, break a Water Jelly Crystal into smaller pieces with your fingers. Compare the results and develop an explanation about what might have happened inside the nuggets. What chemistry took place that allowed the nuggets to grow so large? How did they become so soft in water? What is it that fills the hydrated jelly crystals? Think about your answers on a molecular level and try to explain the changes that you have observed.
How Does It Work
A polymer is a very long chain that’s made up of identical molecules. The molecules act as the links in the chain. Think of these links as tiny sponges waiting to connect with water molecules. In the absence of water, the polymer chain is tightly twisted and coiled, so the connection points are tightly buried inside the nugget. When water is available, the molecular links on the outside of the nugget grab it and hold on through simple cross-linking. The more water that’s available, the more the polymer will unwind to get it. Each link gets larger as it hooks up to more and more water. The chain begins to swell on the outside, which allows more water to get into the polymer to available link points, further expanding. This bonding and swelling continues until there are no more available places to collect water. When absorption stops, the polymer crystals have expanded to about 300 times their original weight. The crystal is now about 98% water and 2% polymer. That’s a lot of water.
Polymers are very common — you see and use them every day: they’re found in silk, wool, nylon, cotton, cellulose, proteins, rubber, PVC, epoxy materials, silicone, hair conditioner, paint, polystyrene, adhesives, gelatin, Silly Putty®, polyester, Kevlar®, CDs, eyeglasses and Teflon®, just to name a few. The list of polymer-containing just goes on and on, kind of like long chains of polymers.
Why should you wash your hands before handling the water jelly crystals? Since the crystal is mostly water, it washes your hands as you handle it. The dirt, germs and microscopic nasties on your hands that it removes have nowhere to go. This buildup can lead to dirty and smelly crystals that are no fun for anyone. Eventually, this oily concoction can cause a reduction in the number of places on the polymer that can hook to water, which means a loss of absorption. Plus, it makes the crystals appear cloudy and dull. Washing your hands before handling the crystals not only prevents dirt from getting into your experiment, but it also helps prolong the life of the crystals and keeps them looking fresh. Plus, it’s just good practice to always wash your hands before and after doing a science experiment.
Take It Further
It’s recommended that distilled water be used to soak the nuggets when possible. Distilled water is usually easy to obtain and is not very expensive. Tap water is okay to use, but sometimes purifying it affects how it gets absorbed by the polymer. Distilled water is like rain water and lacks the minerals, chemicals and other dissolved materials that can change how the polymer bonds with it are removed by distilling, so you get the best, most reliable results by choosing distilled water. Plus, we’ve been told by people doing this experiment that the crystals can grow larger when absorbing distilled water.
Disappearing Crystals: Turn your experiment into a magic trick: the hydrated crystals can vanish if you know the secret. Fill a clear container with plain water and dump four or five of your largest crystals into it. They vanish, but they’re in there. Since they have nearly the same index of refraction as that of the water around them, since they’re 98% water, light passes straight through them and the surrounding water so you can’t see them. If you look very carefully, you can just see the edges of each crystal where light is bent slightly as it passes through the polymer. Check out Vanishing Jelly Marbles for more ideas.
Rainbow Test Tube: Introduce colored crystals into the mix and for a science lab with a creative, artistic twist.
Science Fair Connection
Just Water?: You may be wondering if the polymer can absorb anything other than water. That’s a great question and a great idea for a science fair project. To find out, test any of these liquids instead of water: tomato juice, orange juice, vegetable oil, milk, soda water, rainwater or melted snow, hot water, sugar water and salt water. Keep track of your discoveries and you’re on your way to a science fair project.
Cold Crystals: Place a zipper-lock bag of the hydrated polymer in the freezer. Examine the bag of polymer after 12 hours. Compare the length of chilling time and the temperatures reached for frozen crystals to a similar amount of ice made from regular water. Maybe you could you use frozen crystals the next time you need an ice pack.
Polymer Plants: Grow grass seeds, radishes, beans or other fast-germinating plants in a half-and-half mixture of hydrated polymer and potting soil. Compare by growing the same kind of seeds in plain soil and compare the growth in two-day intervals for one or two weeks. This sounds like another great science fair project. How well do seeds germinate and grow in a cup of hydrated polymer without soil? Test it — you might be surprised.
Safety Information
It’s good advice to keep the crystals away from your mouth, ears, eyes and nose, as usual. However, Water Jelly Crystals are not considered to be a health hazard. They’re non-toxic, safe to handle and safe for use around pets and young children. They’re not supposed to be eaten and can be a choking hazard if used carelessly. Don’t dispose of the crystals, either wet or dry, down the drain, as they will certainly clog the pipe. Instead, toss them into the trash, but remember why they were made: polymers are designed to be environmentally beneficial. These crystals are intended to be used with plants, so when you’re finished using them for discoveries, you have two good options:
- Simply bury the hydrated crystals in a planter box or garden. They dry out as plants use the water they hold and then re-hydrate when water is present in the soil again. They’ll support plant growth for about six years.
- Let hydrated clear or colored crystals completely dry out on a cookie sheet. They shrink back to their original size and can be stored and re-hydrated many times.