
Magic Crystal Tree Kit – Crystal Trees Experiment
Growing a Crystallized Tree – Science Project
Magic Crystal Tree
Impress your friends by creating a colorful Christmas tree out of salt crystals, cardboard and a few other household items. Within a day, you’ll have a colorful, snow-covered tree that magically sprouts from nothing. The Magic Crystal Tree is a fun indoor experiment that can even be used as seasonal decor when you’re done. Complete the project with your child at home using our crystal tree instructions or conduct the experiment with your class at school.
SICK Science® is a registered trademark of Steve Spangler, Inc. All Rights Reserved.
Experiment Materials
- Mrs. Stewart's Bluing (check your local grocer's cleaning section)
- Table salt
- Household ammonia
- Thin cardboard (like the type from the back of a notepad, not corrugated)
- Pen or pencil
- Scissors
- Bowl
- Water
- Measuring spoon
- Food coloring
- Adult supervision
Experiment Videos
Experiment

1
Using a piece of cardboard, trace two Christmas tree shapes and cut them out. Thin cardboard works best for this experiment, rather than corrugated cardboard.

2
Using a sharp pair of scissors, cut a slot down the middle of one cardboard tree. Start at the top and stop in the middle of the shape. Make sure the slot is straight.

3
In the other tree shape, cut another slot down the middle. For this cardboard tree, start at the bottom and cut to the middle. Make sure the slot is straight.
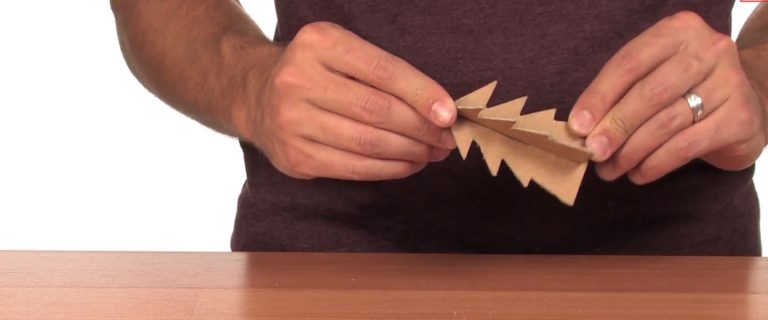
4
Slide the two slots together, creating a three-dimensional tree that can stand on its own.

5
Add drops of food coloring to the edges of the cardboard and let the food coloring soak into the cardboard. Set the tree aside.

6
Using a bowl with a wide mouth or a pie pan, mix the following ingredients together to create the Magic Crystal solution:
- 1 tablespoon of water
- 1 tablespoon of salt
- 1 tablespoon of bluing
- ½ tablespoon of household ammonia

7
Stand your tree in the middle of the bowl containing your solution. Over the next 10 to 12 hours, your Magic Crystal Tree will continue to grow. Watch as crystals form on the cardboard, expanding into beautiful clusters of snow.
How Does It Work
You probably have us figured out. The Magic Crystal Tree isn’t magic at all, but is actually science in disguise. The main principles at work in the Magic Crystal Tree growing experiment are capillary action, evaporation, crystallization and saturation.
Capillary action is the same process that enables plants and trees to absorb water and nutrients from the soil through their stems or trunks and into their leaves, branches, flowers and fruit. The cardboard tree uses the same process to draw up the solution up until it’s entirely soaked.
After the solution has been drawn throughout the tree by capillary action, the solution will begin to evaporate. The process is accelerated by ammonia, which evaporates more quickly than water. As the solution evaporates off of the tree, the crystals are left behind on the branches of the tree. The rapid evaporation helps the crystals form their unique shape.
The crystals that are left behind are a combination of the bluing and the table salt used. The solution that you created in Step 6 is supersaturated by the bluing and salt added to the water. The bluing is a colloid with many tiny particles suspending themselves within the water, which is a lot like a shaken snow globe, except the particles of bluing are much smaller. As the bluing and salt water make their way up the tree, the water begins evaporating. As there is less water to support the bluing particles and dissolved salt, they begin to crystallize, resulting in a Magic Crystal Tree.
Science Fair Connection
Growing crystals on a cardboard tree is pretty cool demonstration, but it isn’t a complete science fair project. You can create a science fair project by identifying a variable, or something that changes, in this experiment. Let’s take a look at some of the variables you can change:
- Change the proportions of ingredients. For example, use some extra salt to see how it affects the growth of the crystals.
- Change the size or shape of the cardboard tree. How does this affect the way that the crystals grow?
- Change the growing conditions. Do the crystals form differently if you grow the tree in a humid environment, like the bathroom?
You aren’t limited to these ideas. Try coming up with different variables to test. Remember, you can only change one thing at a time, or your results may be unreliable. If you are testing different trees sizes, make sure that the other factors remain the same. Write down all of your observations and see if you can figure out which factors determine the growth rate and the size of the crystals. Which combination grows the tree the fastest? What could cause the tree to not grow at all? Use you creativity and come up with a science fair experiment that answers a question you have about crystal formation.
If you like the Magic Crystal Tree experiment, you’ll love the other science experiments at Steve Spangler Science, especially our Water Jelly Crystals experiment.




















