
Boo Bubbles Bouncing Smoke – Dry Ice Bubbles Experiment
Dry Ice Science Experiment
Boo Bubbles Bouncing Smoke Bubbles Experiment
What do you get when you combine dry ice and soap? Ghostly, smoke-filled bubbles, that’s what. For some Halloween fun, learn how to create spooky bouncing bubbles with this hands-on dry ice bubble experiment from Steve Spangler Science. Treat your kids or your class to a hands-on science experiment that will spark their imaginations.
There’s something magical about a bubble. It’s just a little puff of air trapped inside a thin film of soap and water, but its precise spherical shape and beautiful swirling colors make it a true wonder of science. Bubbles are amazing on their own, but bubbles filled with fog are even cooler. Now imagine if you could bounce and play with these bubbles, holding them in your hands without popping.
Our Boo Bubbles are exactly what you get when you fill a bubble with a ghostly carbon dioxide cloud. Boo Bubbles are truly magical because you can roll them on your hands, bounce them off your sleeve and pop them to release a burst of fog. It’s a demonstration of both science and performance art that will have everyone, even you, oohing and ahhing. Get ready for some great fun with this memorable dry ice bubble experiment that will amaze everyone in the room.
SICK Science® is a registered trademark of Steve Spangler, Inc. All Rights Reserved.
Experiment Materials
- Safety glasses *
- Knit gloves *
- Gallon-sized plastic jar *
- 3-foot piece of rubber tubing *
- Liquid soap (Dawn works best)*
- Small plastic container *
- Dish soap *
- Get all the * materials in the Boo Bubble Kit
- Dry ice
- Thick gloves
- Bath towel
Experiment Videos
Experiment

1
Making the Dry Ice Bubble Generator
The Dry Ice Bubble Generator is available as a kit (called the Boo Bubbles Kit) from Steve Spangler Science. It’s a no-hassle option for the person who wants to get started immediately and includes all the materials you need to create amazing dry ice bubbles. It’s also possible to make your own Dry Ice Bubble Generator using items that are commonly found at a department store or in the plumbing aisle of your favorite hardware store.
There are a number of ways to attach the plastic tubing to the jar. You can drill a hole in the jar and attach the hose with a piece of tape or a dab of caulking or glue. The design is up to you, but just remember that whatever design you choose, the jar needs to be roughly 1 gallon in size and the hose needs to be about 3 feet long.

2
Performing the Experiment
Dry ice is cold — really cold. Make sure to take the proper safety precautions when handling dry ice. Start by putting on your safety glasses and some thick leather gloves. These items should protect you from the ice while you perform the experiment. If your ice came in one large piece, as it often does, you might need to use a hammer to break it up into pieces that will easily fit into the jar.
NOTE: You’ll need some thick gloves to handle the dry ice. The knit gloves used later in the activity do not provide enough protection for your hands. Find a good pair of leather gloves to protect your hands against the cold temperature of the dry ice.
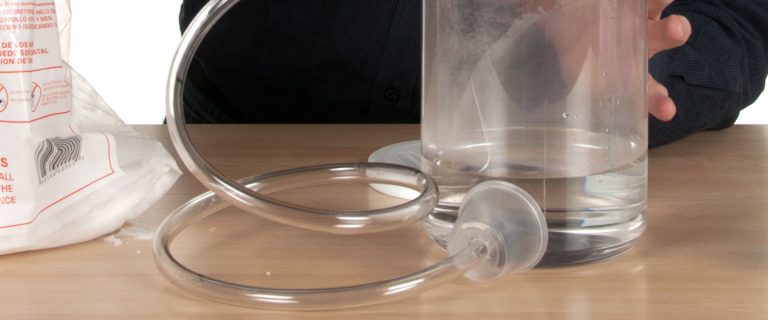
3
Fill half of the jar with warm water. Dry ice produces the best fog when you use warm water. Attach the rubber hose to the side of the jar if it’s not already attached.

4
Drop a few good-sized pieces of dry ice into the jar. The fog will immediately begin to roll out of the jar. Practice covering the top of the jar with the lid to control the flow of fog out of the tube. You don’t have to screw the lid onto the jar, but hold it on top to force more fog through the rubber tubing.
WARNING: Never trap dry ice in a jar without a vent. In other words, there must be a hole in the jar to allow the pressure to escape. Otherwise, the pressure will build up and the jar will explode. This could cause serious harm to you or to someone else.

5
Make a soapy solution by mixing a squirt of liquid dish soap with 4 ounces of water in the small plastic container. Add the water first and then the dish soap to keep it from bubbling up. You’ll be using this solution to make bubbles, but you want the top to be as bubble-free as possible.

6
Dip the free end of the rubber tubing into the bubble solution, wetting the end of the tube. Remove the tube from the bubble solution with one hand while covering the jar with the lid in the other hand. This will take a little practice, but it’s easy once you get the hang of it. The goal is to blow a bubble filled with fog. You won’t have to blow to make these bubbles because the fog from the dry ice will force its way out of the tube to create a bubble for you.

7
When the bubble reaches the perfect size, gently shake it off of the tubing. Your bubble will be heavier than a normal bubble because it’s filled with carbon dioxide gas and water vapor, so it will quickly fall to the ground. When the bubble hits the ground, it will burst and a cloud of fog will erupt from the bubble.
How Does It Work
When blowing bubbles indoors, you may have noticed the occasional bubble that fell to the carpet but didn’t pop. Regular bubbles burst when they come into contact with just about anything. A bubble’s worst enemies are oil and dirt, so soap bubbles will bounce off of a surface if it is free of oil or dirt particles that would normally puncture the soap film. This means they won’t break if they land on a softer fabric, like gloves or a towel.
Dry ice is frozen carbon dioxide. Instead of melting, dry ice turns directly into carbon dioxide gas. It doesn’t melt like normal ice because it skips the liquid stage and goes straight from a solid to a gas in a process called sublimation. When you drop a piece of dry ice in a bucket of water, the gas that you see is a combination of carbon dioxide and water vapor. So, the gas is actually a cloud of tiny water droplets.
Grocery stores use dry ice to keep food cold during shipping. Some grocery stores and ice cream shops will also sell dry ice to the public, especially around Halloween. Dry ice comes in flat square slabs a few inches or centimeters thick or as cylinders that are about 3 inches (76 millimeters) long and about 1/2 inch (13 millimeters) in diameter. Either size will work fine for this experiment.
Take It Further
Touchable Boo Bubbles
This Boo Bubble variation happened accidentally at Steve Spangler Science and has become a must-do experiment. A bath towel was stretched out on the table to make the cleanup a little easier and to everyone’s amazement, some of the fog-filled bubbles bounced on the towel and didn’t pop. It just goes to show you how educational playtime can be. It’s important to mention that not all types of fabric behave the same way, so you can test some hypotheses about which materials are best for preserving bubbles.
If fog-filled bubbles will bounce on a towel, what would happen if you wrapped your hands in fabric and tried to touch or play with the bubbles? You can easily find out by purchasing a pair of inexpensive knit gloves. Blow a baseball-sized bubble with our Dry Ice Bubble Generator and bounce the bubble off of your gloves. Next, try bouncing the bubble on your shirt or pants and see what happens with a different fabric. Start up a game of bubble volleyball with a friend who’s looking for some creative fun.
Giant Boo Bubbles
Regular-sized Boo Bubbles are awesome, but giant Boo Bubbles are even more awesome. All you need are a few extra parts from around the house. Cover a clean surface with a thin layer of soap bubble solution and spread it around. Fill the large water bottle with warm water and drop in a few big pieces of dry ice. Again, never seal the bottle completely because the rapidly expanding gas could result in an explosion.
Hold the large plastic hose over the top of the large water bottle. The fog will start flowing out of the hose. Dip the open end of the hose into the bubble solution and put it down on the soap-covered table. A giant Boo Bubble will start forming on the surface of the table. Keep the nozzle down and your bubble will continue to grow. When the bubble finally pops, all of that carbon dioxide gas will escape, leaving a ghostly fog behind.
Try the Boo Bubbles experiment at home or take it to the classroom for a large-scale demonstration that young scientists will love. Boo Bubbles are easy and inexpensive to make and are a great introduction to states of matter.
Science Fair Connection
WANT MORE SCIENCE EXPERIMENTS?
Love science experiments? Sign up for the Steve Spangler Science Club and we’ll send you a everything you need to perform an awesome experiment in your very own home every month.
Safety Information
SAFETY INFORMATION
NOTE: Whenever you use dry ice, always be aware of the rules for handling it safely:
•Dry ice is not a toy. It’s for demonstration purposes only.
•Use dry ice only with adult supervision.
•Dry ice must be handled using heavy gloves or tongs. It will cause severe burns if it comes into contact with bare or unprotected skin.
• Always wear safety goggles when handling dry ice. The debris and shards are extremely dangerous to your eyes. When tapping dry ice with a hammer to break it, first cover the dry ice with a towel to keep the pieces in one place.
• Never put dry ice in your mouth.
•Never store dry ice in an airtight container. As the dry ice sublimates, gas pressure will build, and the container will explode. Make sure your container is ventilated or has a loose-fitting lid.
• Do not store dry ice in your freezer. It will cause your freezer to become too cold and the freezer may shut off. On the other hand, if you lose power for an extended period of time, dry ice (if you can get it) is a good way to keep things cold.
•In the unlikely event of a dry ice burn, treat it the same as you would a heat burn. See a doctor if the skin blisters or comes off. Apply antibiotic ointment to prevent infection and bandage mild burns.
What It Dry Ice?
Remember that dry ice is not frozen water, it’s frozen carbon dioxide (CO2). Unlike most solids, dry ice does not melt into a liquid as its temperature rises. Instead, it changes directly into a gas. This process is called sublimation. The temperature of dry ice is -109.3°F (-78.5°C). Dry ice is particularly useful for keeping things cold because of its temperature. Dry ice does not last very long, however, so it’s important to purchase the dry ice you need for these science activities as close as possible to the time you need it. The best place to store dry ice is in a Styrofoam ice chest with a loose-fitting lid that allows the CO2 to escape as the ice sublimates.
Remember the science when purchasing dry ice: dry ice in a grocery bag will vanish in about a day. The experts tell us that, depending on weather conditions, dry ice will sublimate at a rate of five to 10 pounds (2.3 to 4.5 kg) every 24 hours, even in a typical Styrofoam chest. So, again, it’s best to purchase the dry ice as close to the time you need it as possible. Last minute shopping is necessary. If you are planning to perform several dry ice demonstrations or have a lot of people involved, purchase five to 10 pounds (2.3 to 4.5 kg). A little dry ice does go a long way in these activities.
How It’s Made
The first step in making dry ice is to compress carbon dioxide gas (CO2) until it liquefies, while at the same time removing excess heat. The CO2 will liquefy at a pressure of approximately 870 pounds per square inch (4500 cmHg) at room temperature. Once liquid CO2 is formed, the CO2 is sent through an expansion valve and enters a pressure chamber. This pressure change causes the liquid to flash into a solid and causes the temperature to drop quickly. About 46% of the gas will freeze into “dry ice snow.” The rest of the CO2, about 54%, is released into the atmosphere or recovered to be used again. The dry ice snow is collected in a chamber where it is compressed into block, pellet or rice-sized pieces with the use of hydraulics. It’s complicated but really cool science. Really cool!
Can you make your own dry ice? Sure, anything is possible. It’s not practical, though — unless you have a huge tank of compressed CO2 sitting around and lots of extra time and equipment on your hands. For around $2 USD a pound, it’s hard to beat the convenience of just purchasing it at the store.
Boo Bubbles and More!
Can’t get enough of dry ice bubble experiments? Don’t miss our other super-fun dry ice activities that are great for learning about chemical reactions and sublimation. We’ve compiled some great dry ice ideas that can be performed at home or in the classroom. These ideas are also great for Halloween party activities and some atmospheric fun. Speaking of Halloween parties, be sure to check out our amazing ideas for the best Halloween party ever, which includes exploding pumpkins, dry ice experiments, fake blood and some other screaming good party ideas that will leave your guests amazed.




















